| PURPOSE:
To give the student experience in new laboratory equipment and to observe the process of acid rain production.
INTRODUCTION:
"Acid rain" is a broad term referring to a mixture of wet and dry deposition (deposited material) from the atmosphere containing higher than normal amounts of nitric and sulfuric acids. The precursors, or chemical forerunners, of acid rain formation result from both natural sources, such as volcanoes and decaying vegetation, and man-made sources, primarily emissions of sulfur dioxide (SO2) and nitrogen oxides (NOx such as NO2) resulting from fossil fuel combustion. In the United States, roughly 2/3 of all SO2 and 1/4 of all NO2 come from electric power generation that relies on burning fossil fuels, like coal. Acid rain occurs when these gases react in the atmosphere with water, oxygen, and other chemicals to form various acidic compounds. The result is a mild solution of sulfuric acid and nitric acid. When sulfur dioxide and nitrogen oxides are released from power plants and other sources, prevailing winds blow these compounds across state and national borders, sometimes over hundreds of miles.
During this lab you will generate SO2 and NO2 and produce your own acid rain.
---*---

|
pH paper is supplied through the dispenser shown to the left. |
---*---
|
The gases produced are contained in a special gas collecting bottle.
|
|
---*---
|
A deflagrating spoon, is used to hold burning sulfur. (a hand will assist) |
---*---
|
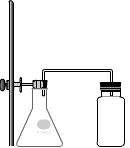
The set up shown here is used to collect nitrogen dioxide.
|
|
---*---

|
If you get into trouble during the lab you can start over by pressing the reset button. |
PROCEDURE:
1)
Click on the "Special"
button and select "Print Blank Report" to obtain a web page that
can be printed and used as a lab report. (the program will not be interrupted)
Room 1: SO2
2) Put your goggles on. Turn on the hood light by clicking on the light switch found on the right of the hood.(the venting fan is already on) Grab the deflagrating spoon found in the gas collecting bottle(a hand will assist), and move it up and over to one side. Pick up the red water bottle and hold its spout over the opening to the gas collecting bottle. Press 'p' to squirt in a sample of water. This water will eventually turn into our acid rain. Pick up a piece of pH paper and dip it in the water in the bottle. Record the pH shown on the pop-up pH scale.
3) Pick up the forceps(tweezers) holding a piece of sulfur and center the sulfur lump just above the bowl of the deflagrating spoon. Press 'p' to release the sulfur so it falls into the spoon.
4) Click on the gas lever found far to the left of the hood to turn on the gas. Pick up a match and hold it to the top of the bunsen burner to light the flame. Move the deflagrating spoon over so the bowl of the spoon is in the flame. The sulfur should start to burn with a blue flame. Turn off the burner and place the deflagrating spoon in the gas collecting bottle so the horizontal shield rests on the top of the bottle.
5) The SO2 gas is now formed from the combustion of sulfur. When all of the oxygen is used up in the bottle and the sulfur flame goes out, remove the deflagrating spoon. Dip another piece of pH paper into the water in the bottle. Record the pH shown on the pop-up pH scale.
6) Place the black stopper in the mouth of the bottle, pick up the bottle and shake it, mixing the gas and air. Remove the stopper and Record the pH of the water in the bottle. This room is done, move on to the next room by pressing the green button on the right side of the hood.
Room 2: NO2
7) Squirt water into the gas collecting bottle and test with a piece of pH paper. Record. Place the rubber stopper in the mouth of the bottle.
8) Pick up the small graduated cylinder, containing nitric acid, and hold its left lip centered over the mouth of the empty flask. Press 'p' to pour in the acid. Pick up the piece of copper metal on the table to the left and drop it into the flask. NO2 is now being generated! Drag the glass tubing, clamped to the stand on the left, downwards until it pops into place on the flask. The gas will now be directed over to the gas collecting bottle. Wait until the reaction stops.
9) Raise the glass tubing up out of the way. Remove the rubber stopper from the gas collecting bottle and test the pH of the water as before. Record.
10) Put the stopper back in the bottle, shake and test this pH also. Record.
11)
Answer the questions asked for on the lab sheet and any given
by your teacher. For
help on these values click on the "Special" button and select
"View Data & Hints". Select "File Report" to send a copy
of this lab to be viewed by your teacher.
|